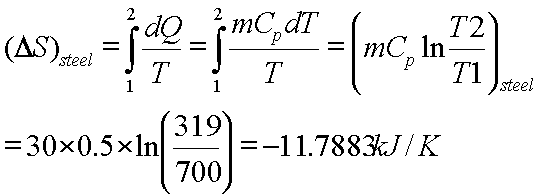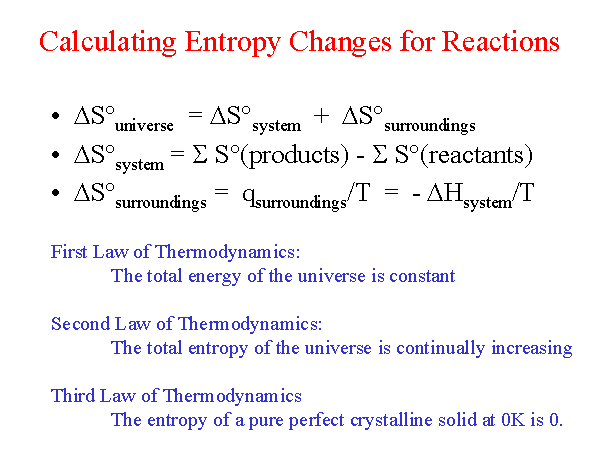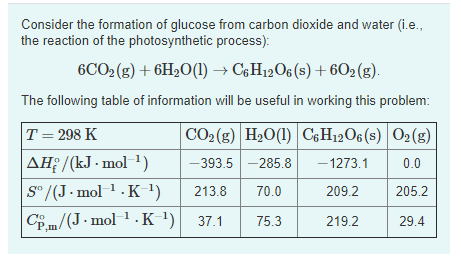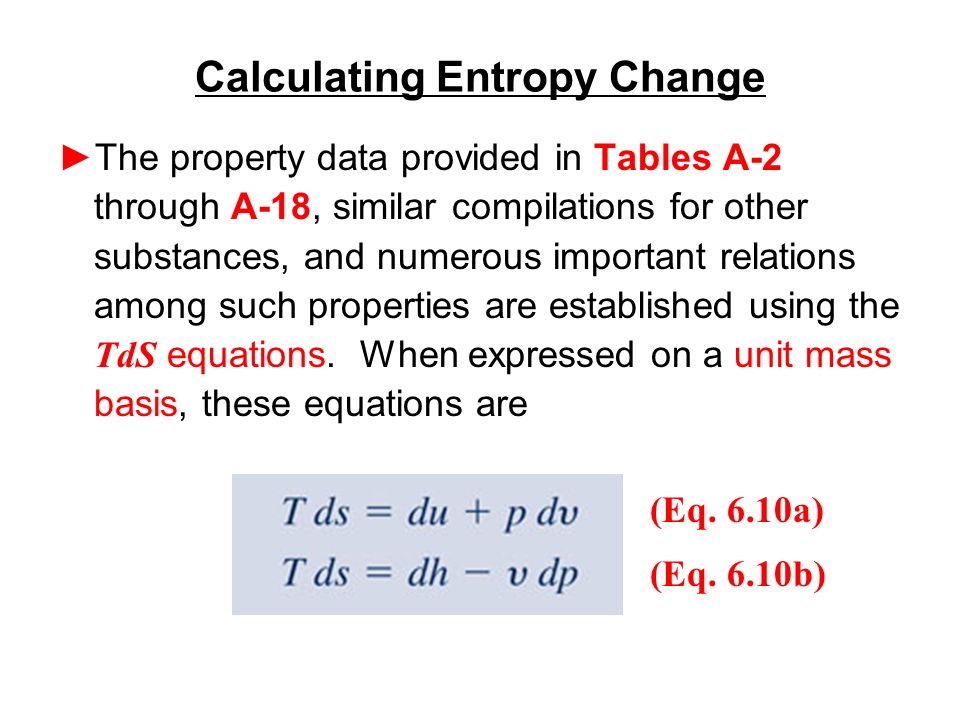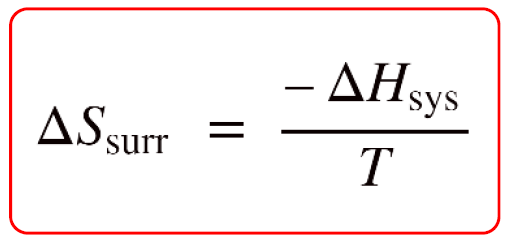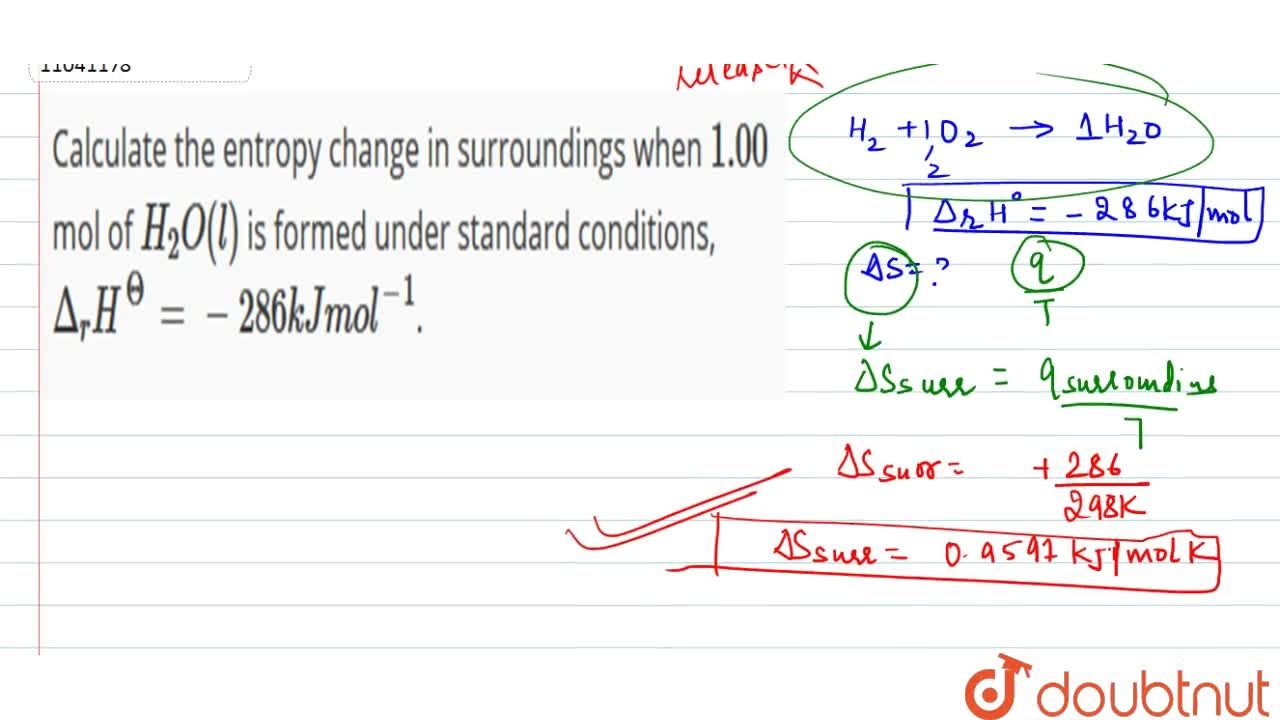
Calculate the entropy change in surroundings when 1.00 mol of H(2)O(l) is formed under standard conditions, Delta(r )H^(Θ) = -286 kJ mol^(-1).

Calculate the entropy change in surrounding when 1.00 mol of H2O(l) is formed under standard condition fH^ = - 286 KJ mol^-1 .
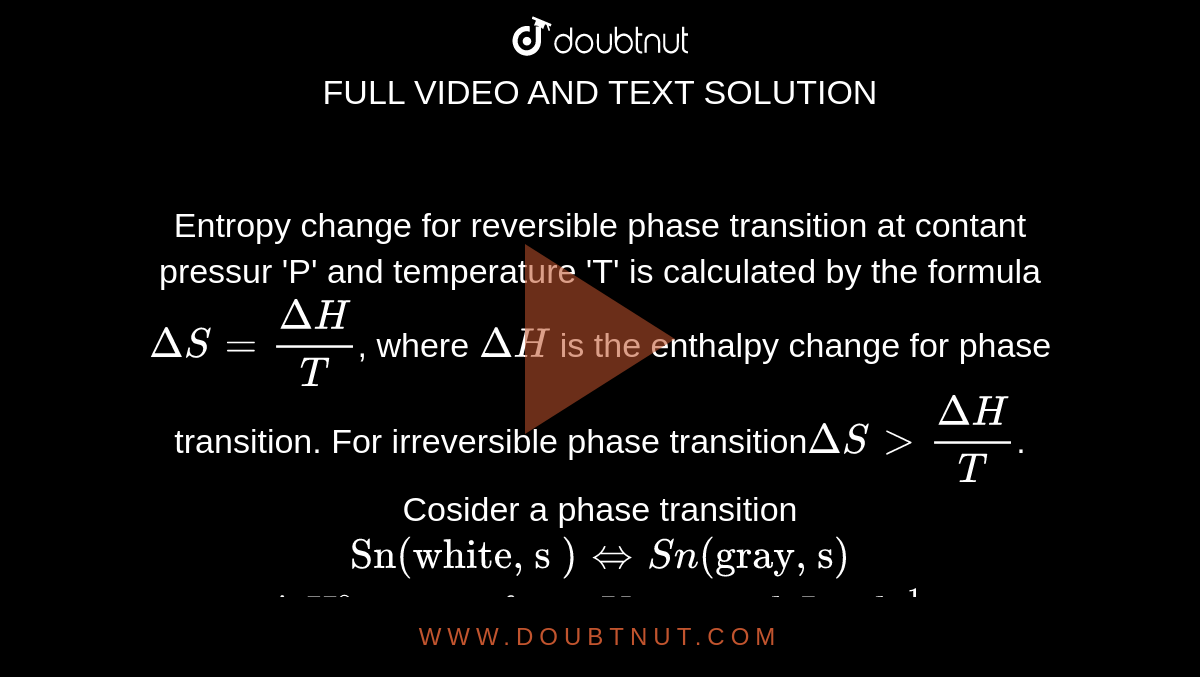
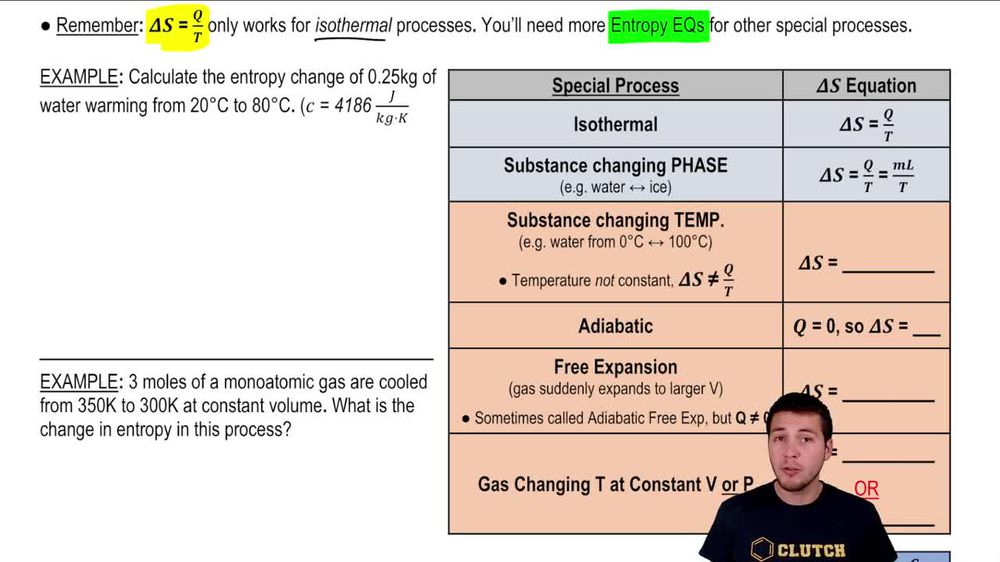
Entropy change for reversible phase transition at contant pressur 'P' and temperature 'T' is calculated by the formula DeltaS=(DeltaH)/(T), where DeltaH is the enthalpy change for phase transition. For irreversible phase transitionDeltaS

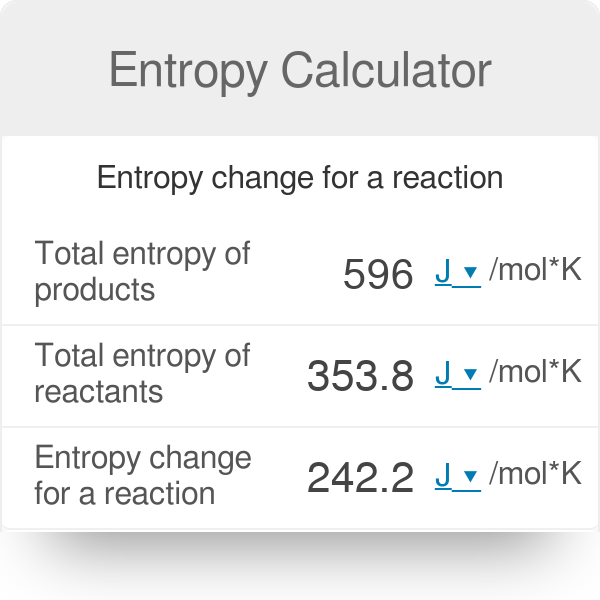
How to Calculate the Entropy Change for a Chemical or Physical Process Based on Absolute Entropies | Chemistry | Study.com

SOLVED: Calculate the entropy change of the universe (J/mol-K) when the entropy change of the system is 59.4 J/mol-K and the surroundings absorb 33.71 kJ of heat from the system at 77.74 °